Abstract
Background Deferasirox (DFX) is an oral iron chelator widely employed in the clinical practice to reduce iron overload in patients with myelodysplastic syndromes (MDS); at present, however, there is no specific report on the treatment with DFX in very elderly patients, who represent an important fraction of MDS subjects
Aim To appreciate safety and efficacy of DFX treatment in very elderly MDS patients, comparing those treated when older than 75 years with younger subjects as to efficacy and toxicity
Methods We evaluated a retrospective cohort of 150 consecutive MDS patients of any age followed in 9 hematological Centers in Italy: sixty-eight patients were older than 75 years and 82 younger when DFX was started. The main features at diagnosis according to age at baseline of DFX treatment are reported in the Table 1: younger patients had a higher rate of high-risk MDS, with lower PLTs values and higher ferritin levels at diagnosis, before any transfusion support
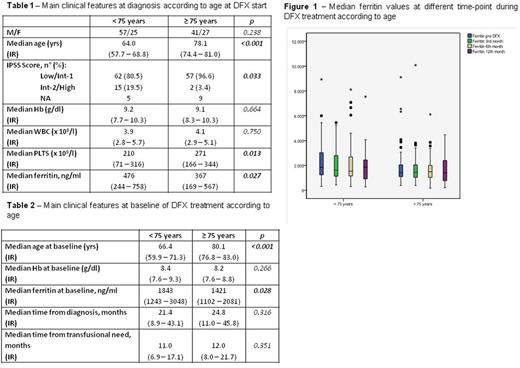
Results The main features at baseline of DFX treatment according to age are reported in the Table 2. Median starting DFX dose was 1,000 mg/day in both groups (p=0.545). As to toxicity, 26/82 patients (31.7%) in younger group vs 32/68 (47.0%) in older group (p=0.061) presented grade ≥ 2 WHO adverse events: a temporary discontinuation of DFX treatment due to toxicity was needed in 16/82 (19.5%) younger patients compared to 15/68 (22.0%) older patients (p=0.552), while a permanent discontinuation of DFX due to toxicity was needed in 8/82 (9.7%) younger patients compared to 13/68 (19.1%) older subjects, with a trend of significance (p=0.056). In both groups, the median values of ferritin remained stable between the baseline and the 12th month (from 1843 to 1858 ng/ml in younger patients; from 1421 to 1413 ng/ml in older patients), notwithstanding the stable transfusion burden in the same period: in the figure 1, median values of ferritin are shown at different time-points during the 1st year of treatment. Median overall survival from DFX start was similar in the 2 groups [62.0 months (95%CI 31.4 - 92.6) in younger patients vs 52.8 months (95%CI 29.3 - 76.2) in older patients, p=0.324)
Conclusions Treatment with DFX is feasible and effective also in very elderly patients with MDS and iron overload, with results and toxicity quite similar to younger patients.
Galimberti: Pfizer: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau. Breccia: Pfizer: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Incyte: Consultancy. Foà: Amgen: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Sandoz: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal